Approximately 70% of ovarian cancers are said to be "high-grade serous"[2] and have a severe prognosis. Optimizing treatments and developing new strategies therefore represent major scientific and medical challenges. Today, scientists are interested in a specific cell population: fibroblasts, heterogeneous cells present throughout the body at the level of connective tissues (which ensure the cohesion of the other tissues of the organism). Cancer-associated fibroblasts (CAF) participate in maintaining the tumor microenvironment, considered an important driver in the development of the disease (for example, by promoting the spread of metastases). Understanding the role and evolution of CAF during the disease and its treatment is therefore essential to find more effective therapeutic approaches against cancer.
The effect of chemotherapy on fibroblasts associated with cancer
Within the Cancer, Heterogeneity, Instability and Plasticity Unit (Inserm, Institut Curie), the team led by Dr Fatima Mechta-Grigoriou[3], evaluated the effect of chemotherapy treatment on four previously identified CAF populations in high-grade serous ovarian cancers. The researchers managed to distinguish several categories of CAF: some, beneficial, would block tumor development, while others, harmful, would participate in the growth of cancer. Scientists have observed that a significant proportion of harmful CAF is inactivated as a result of chemotherapy treatment. However, a variable proportion of these harmful CAF remains activated despite chemotherapy, with an impact on the effectiveness of the treatment.
CAF and the Immune System
The team wanted to go further and was interested in the interaction between these harmful CAF and the immune system. Its results reveal that the population of harmful CAF blocks the antitumor activity of essential immune cells: CD8 T lymphocytes+. Thus, targeting these harmful residual CAF, in combination with chemotherapy, could improve the prognosis of patients with ovarian cancer.
"These results suggest that a therapeutic approach specifically targeting these residual harmful CAFs, in addition to chemotherapy, could increase the antitumor activity of CD8 T lymphocytes+ and improve the treatment of cancer and the prognosis for patients," explains Dr. Fatima Mechta-Grigoriou. "At Institut Curie, we are currently conducting the CASSIOPEIA University Hospital Research Project, which focuses on these same CAF populations to fight against metastases and resistance to treatment in triple negative breast cancers."
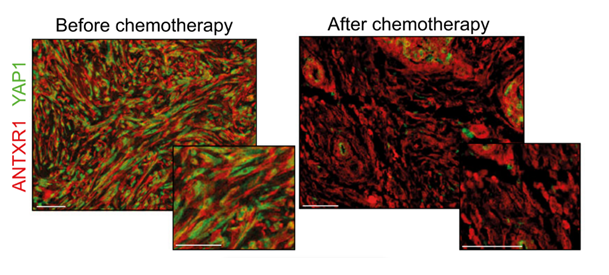
Labeling of harmful CAF populations (ANTXR1, in red) and intracellular YAP1 proteins (in green) in cells before and after treatments. After chemotherapy, the residual presence of YAP1 in green is observed, which could be targeted to increase the effectiveness of chemotherapy. Scale bars, 50 µm
Fibroblasts beyond cancer
Recently published results[4] by the same team have also highlighted a role of fibroblasts in the development of chronic kidney diseases, a major cause of mortality in the world. By accumulating, these fibroblasts induce renal dysfunctions. The scientists have thus shown that the presence of particular fibroblasts at the diagnosis is predictive of an unfavorable prognosis in the patient.
"The various populations of fibroblasts are involved at different stages of pathological development in cancer, but also appear in new and intriguing ways in other pathologies, which greatly expands our field of research," concludes Dr. Fatima Mechta-Grigoriou.
|
Citation: Monika Licaj (…) and Fatima Mechta-Grigoriou. Residual ANTXR1+ myofibroblasts after chemotherapy inhibit anti-tumor immunity via YAP1 signaling pathway. Nature Communications, 2024 Feb 12;15(1):1312. doi: 10.1038/s41467-024-45595-3. |
[1]Tumor microenvironment is defined as all the cells or biological constituents (blood vessels, immune cells, fibroblasts, signaling molecules, extracellular matrix) that are located around the cancer cells and that interact strongly with them.
[2] High-grade serous ovarian cancer is a subtype that develops from epithelial cells.
[3] The Stress and Cancer team at Institut Curie is led by Dr. Fatima Mechta-Grigoriou, the Director of Exceptional Research at Inserm.
[4] Cohen et al., 2024, WNT-dependent interaction between inflammatory fibroblasts and FOLR2+ macrophages promotes fibrosis in chronic kidney disease, Nature Communications, 2024 Jan 25;15(1):743. doi: 10.1038/s41467-024-44886-z.