Ultraviolet rays, alcohol, tobacco, genetic predisposition, spontaneous mutations... so many factors constantly damage our genome. Among these lesions, breaks that affect both DNA strands simultaneously are the most harmful. Our body is constantly repairing this damage through several repair systems, including homologous recombination. However, when these mechanisms fail (due to a genetic mutation, for example), they may cause cancer to occur. Also, the proven correlation between these homologous recombination deficiencies and the aggressiveness of cancers or their resistance to current chemotherapies underlines the pressing need for targeted cancer-fighting therapies.
A new major player in DNA repair: PolꝊ
A few years ago, a new player in DNA repair (polymerase theta or PolꝊ) was identified as a therapeutic hope for treating these cancers[1]. The “Alternative DNA Repair Mechanisms in Human Cancers”[2] team headed by Dr. Raphaël Ceccaldi, Inserm researcher at Institut Curie, has just clarified the mechanism for the action of this polymerase and the reason why this enzyme is vital to the development of breast and ovarian cancers.
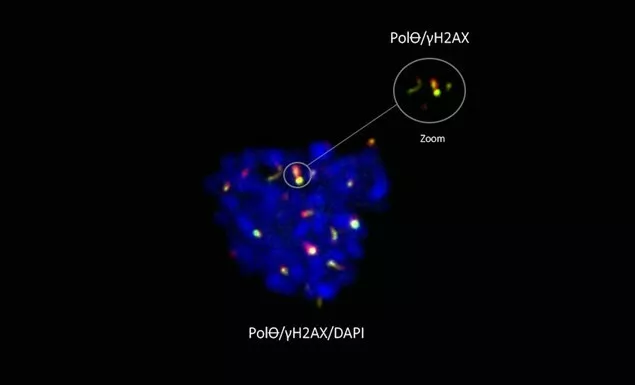
For the first time scientists have shown that PolꝊ works where other DNA repair pathways do not. While it was believed that DNA repair was impossible during cell division (when DNA is extremely compacted), the team from Institut Curie showed that PolꝊ is active specifically during mitosis when the other contributors to repair were proven ineffective.
“Along with my team at Institut Curie, we looked closely at the mechanisms that the cell puts in place to repair its DNA, enabling cancer cells to survive. It is by understanding such mechanisms that we can build new options to thwart cancer“, explains Dr. Raphaël Ceccaldi, Inserm researcher and team leader at Institut Curie. “Our discoveries on the role and functioning of polymerase theta in maintaining integrity of the genome gives us hope for new therapeutic strategies against cancer, particularly breast and ovarian cancer.“
Genome integrity highly preserved by PolꝊ
Through a collaboration with the team of Dr. Sophie Zinn-Justin, researcher at the CEA (Laboratoire de Biologie Structurale et Radiobiologie), researchers went even further, showing that, in order to repair DNA, PolꝊ had to be activated by an enzyme specifically present during cell division. In addition, the mechanisms that enable this activation of PolꝊ seem to have been extremely well-preserved throughout evolution. This suggests that they play an important role in maintaining the stability of the genome needed for development of eukaryotic organisms.
A therapeutic hope for breast and ovarian cancer
The team of Dr. Raphaël Ceccaldi also discovered that inhibiting PolꝊ during cell division by mitosis prevents the proper repair of DNA and as a result leads to the death of cancer cells. With almost half of breast and ovarian cancers showing DNA repair deficiencies by homologous recombination, this step therefore represents a milestone in the fight against these cancers. Clarifying the molecular mechanisms governing the use and regulation of PolꝊ could ultimately lead to the development of new therapeutic targets for treating these cancers.
[1] Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin M, O’Connor KW, Konstantinopoulos PA, Elledge SJ, Boulton SJ, Yusufzai T, D’Andrea AD. Homologous recombination (HR)-deficient tumors are hyper-dependent on POLQ-mediated repair. Nature. 2015 Feb 12;518(7538):258-62.
[2]Cancer, Heterogeneity, Instability and Plasticity unit - CHIP (U830, Institut Curie/Inserm)
Reference: Polθ is phosphorylated by PLK1 to repair double-strand breaks in mitosis. Camille Gelot, Marton Tibor Kovacs, Simona Miron, Emilie Mylne, Alexis Haan, Liza Boeffard-Dosierre, Rania Ghouil, Tatiana Popova, Florent Dingli, Damarys Loew, Josée Guirouilh-Barbat, Elaine Del Nery, Sophie Zinn-Justin & Raphael Ceccaldi. Nature – 6 September 2023 - https://www.nature.com/articles/s41586-023-06506-6
Legend above : PolꝊ (green) marks DNA breaks (gH2AX, red) in the mitotic chromosomes (DAPI, blue) – Scale 5μM