Medulloblastoma is the most common brain cancer in children. It develops in the cerebellum and belongs to a very heterogeneous group of tumors. Among these tumors, Group 3 medulloblastoma is poorly characterized and is among the most aggressive. Unlike adult cancers that result from aging and exposure to mutagens, pediatric cancers emerge from dysregulation of the developmental programs that govern normal cell fate decisions.
Medulloblastoma develops from a fetal region in the cerebellum, however, until now the precise cell at the origin of these tumors was not known. Today, the Genomics and Development of Childhood Cancer Team of Dr. Olivier Saulnier at Institut Curie (Cancer, Heterogeneity, Instability and Plasticity unit - CHIP U830 (Inserm, Institut Curie)), in collaboration with his Canadian colleagues, unveil the mechanisms at the origin of group 3 medulloblastoma, in particular the cellular and molecular mapping of this cell type during early human embryogenesis.

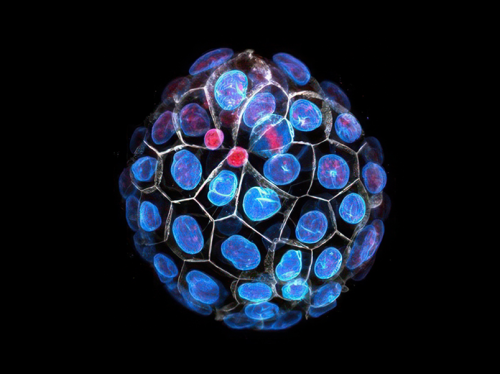
Legend: Oncogenic ballet: cellular heterogeneity of medulloblastoma in the cerebellum: It is possible to distinguish the stem-like cells (tumor initiating cells in yellow) from their progenitor cells (in green) thanks to the presence or absence of the PRTG marker. Tumor cells are sustained by endothelial cells, which ensure nutrient exchanges between the vascular system and tumor cells thus allowing maintenance and proliferation.
The cell of origin unmasked
By tracking stem cells and their progeny in human embryonic tissues, the researchers revealed the detailed characteristics of the cell at the origin of group 3 medulloblastoma, so-called cell of origin. The precise identity of this cell has been determined via the use of bioinformatics making it possible to study genomic data at the single cell level. By comparing the tumor programs with the human embryonic developmental programs, the scientists managed to identify that the cell of origin expresses an embryonic marker named Protogenin (PRTG). During human development, this marker is expressed only during the first weeks of pregnancy and is therefore not expressed during adult life. This fetal "fingerprint" is very important because thanks to it, it is possible to imagine new therapies targeting the cells at the origin of these tumors.
This is what the researchers have demonstrated: targeting PRTG-expressing cells reduce tumor growth and increase survival in vivo in pre-clinical models of medulloblastoma.
"By identifying this onco-fetal marker in very young children, we could imagine preventing the disease even before it develops and therefore blocking the process of tumorigenesis. This work will have considerable implications in the fight against various pediatric cancers, opening up new avenues of research that could be testable in other types of tumors," explains Dr. Olivier Saulnier, Group Leader of the Genomics and Childhood Cancer Development team
Towards new therapeutic weapons against group 3 medulloblastoma
By targeting exclusively the cell of origin, which is now identified, it is now possible to stop tumor development while sparing non-aggressive cells. Several innovative therapeutic approaches are being evaluated. These results make it possible to design the development of new therapies such as antibodies targeting specifically PTRG protein, which is expressed only on the surface of the "daughter" cells descending from the cell at the apex of medulloblastoma hierarchy. Another option could be the use of cell therapy (CAR-T). Thus, by modifying the receptors on the surface of certain white blood cells, the T lymphocytes of an individual's immune system, they are specifically trained to fight the cells expressing PRTG protein and then reinjected into the same individual and thus killing the tumor cells.
"In addition to targeted therapies or innovative cell therapies, we are investigating other avenues in order to develop more effective and less invasive treatments for young patients. We are working on understanding the mechanisms ensuring the maintenance of this cell population during post-natal life, thus preventing the development of full-blown medulloblastoma tumors," concludes Dr. Olivier Saulnier.
|
References: > Early Rhombic Lip Protogenin+ve Stem Cells in a Human Specific Neurovascular Niche Initiate and Maintain Group 3 Medulloblastoma. Abhirami Visvanathan*, Olivier Saulnier*, Chuan Chen* (...) Dimiter S Dimitrov, Michael D. Taylor. Cell, July 5, 2024. DOI: https://doi.org/ 10.1016/j.cell.2024.06.011
[1] This work was carried out thanks to the collaboration of Institut Curie, The Hospital for Sick Children (Toronto, Canada), Baylor College of Medicine (Houston, USA), the University of Pittsburg (USA) *: first co-authors The work carried out by the team of Dr. Olivier Saulnier, Junior Principal Investigator at Institut Curie, was made possible thanks to the financial support and generosity of the public. |
Figure : © Institut Curie / Olvier Saulnier
Photo : © Institut Curie / Alexandre Darmont