Immunotherapy is an anti-cancer strategy that has been creating a buzz for around fifteen years. In receptive patients it gives spectacular results, but 50 to 80% of patients do not respond to these treatments. At Institut Curie, the Innate Immunity team of Dr. Nicolas Manel, Inserm research director, has just developed an innovative strategy that could be a game changer for these patients.
Dr. Manel uses a mechanical metaphor to explain how the current so-called anti-checkpoint immunotherapies work: “These therapies lift the immune system’s “brake” so that it recognizes the patient’s cancer cells as cells to be destroyed (without these anti-checkpoints, the immune system cannot attack the cancer cells). But they work only if there is a “natural accelerator” so that the immune system attacks these cells. This accelerator is linked to a biochemical pathway known as STING and it is missing in 50 to 80% of patients.”
Understanding the basic mechanisms
It was around five years ago that researchers understood the accelerating role of STING. Pharmaceutical laboratories immediately developed small molecules to activate STING. Although persuasive in vitro, their results proved disappointing in a clinical setting.
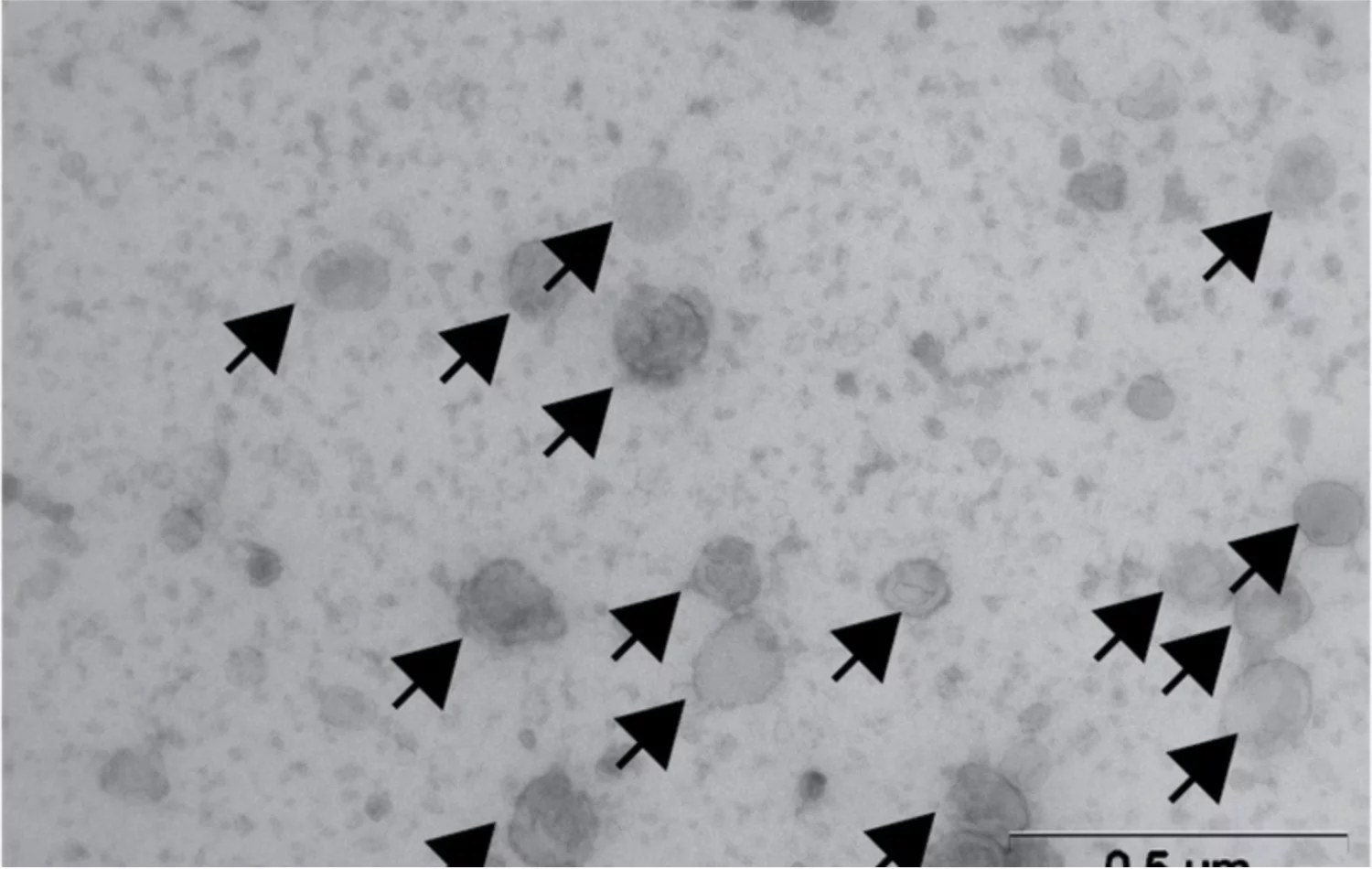
At Institut Curie, Dr. Nicolas Manel and his team got back to basics to decipher the entire cascade of events at play: “What our study reveals is that STING must be activated selectively in certain cells, the dendritic cells, to have an accelerator effect, whereas activating STING in other cells can destroy the immune response.” The researchers used pseudoviral particles (which resemble viruses but are not infectious) since they are efficiently captured by the dendritic cells. They then placed the natural molecule that activates STING - known as cGAMP - inside these particles. The drug thus obtained, known as cGAMP-VLP, activates STING preferentially in the dendritic cells, without negative impact on the other cells of the immune system. These activated dendritic cells have thus educated the lymphocytes so that they recognize the tumor’s antigens. Hence, the researchers identified a very much sought-after therapeutic product, which is capable of activating the accelerator.
“Tested in various systems (in vitro, in animal models and on human tumor samples), this strategy produced a strong anti-tumor effect, even at low dose. Combined with an anti-checkpoint immunotherapy, it is even more effective,” declares Dr. Manel. He believes it’s therefore time to offer this new therapeutic tool to patients. This is the goal of Stimunity, the biotechnology company that it co-founded, which, with the current fundraising efforts, could launch the clinical development of this innovative immunotherapy.
If these results are conclusive in humans, it will be possible to attack these cancers that until now have been resistant to all known treatments, without using chemotherapy or radiotherapy.
Référence :
Selective STING stimulation in dendritic cells primes anti-tumor T cell responses. Bakhos Jneid, Aurore Bochnakian, Caroline Hoffmann, Fabien Delisle, Emeline Djacoto, Philémon Sirven, Jordan Denizeau, Christine Sedlik, Yohan Gerber-Ferder, […] et Nicolas Manel. Science Immunology. January, 13 2023. doi:10.1126/sciimmunol.abn6612