|
Institut Curie experts at ESMO From September 13 to 17 in Barcelona, Spain, the world cancer community will gather for the congress of the European Society of Medical Oncology. For five days, doctors and researchers, including those from Institut Curie, will share their very promising results and their expertise to accelerate the fight against cancer on all fronts. |
"While immunotherapy has already transformed the care of our patients in recent years, many avenues are being explored, particularly at Institut Curie, to relentlessly optimize these therapeutic strategies. In this sense, the positive results that we are presenting at ESMO foreshadow the immunotherapy of tomorrow and show us that we must continue this strategy of acting on several pathways to stimulate the immune system," says Prof. Nicolas Girard. "We will now launch the phase 3 study to evaluate this combination of immunotherapies in patients with a common form of metastatic lung cancer involving more than 15,000 people per year in France."
The leading cause of cancer-related death worldwide, with the highest mortality rates in both men and women, lung cancer affects nearly 53,000 people per year in France. The most common form is non-small cell lung cancer. Very often, this pathology is diagnosed at an advanced stage, when treatment options are limited. While primary prevention and screening of at-risk populations are crucial issues in the fight against this scourge, it is also essential to continue to develop new therapeutic strategies to treat patients in the best possible way.
Proof-of-concept Ph2 trial identifies potential LAG-3 responder population in metastatic NSCLC
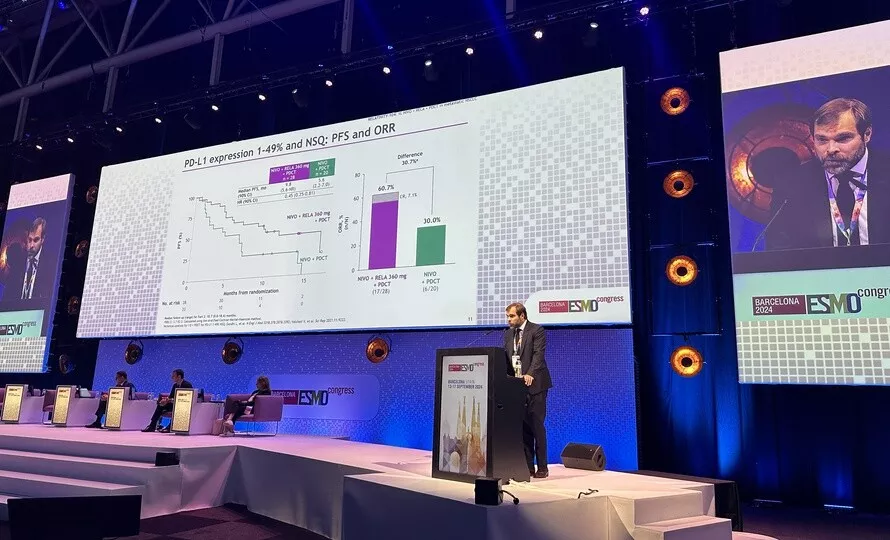
Currently, the general standard treatment for metastatic non-small cell lung cancer is chemotherapy combined with immunotherapy (an antibody against the PD-1 protein, the immune system checkpoint). There were two key objectives of the randomized, proof-of-concept, RELATIVITY-104 phase 2 clinical study:
- Investigate the clinical benefit of adding LAG-3 inhibition (relatlimab) on top of current current standard-of-care anti-PD-1 immunotherapy plus chemotherapy
- Identify potential LAG-3 responder populations within 1L metastatic NSCLC
Relatlimab (anti-LAG-3 antibody) is one of the new approaches in immunotherapy. The LAG-3 receptor is expressed on the surface of certain immune cells (T cell type). It facilitates the binding between cancer cells and T lymphocytes, and prevents the anti-cancer action of the latter. In fact, by blocking the LAG-3 receptor, relatlimab can significantly improve the action of the immune system against the tumor.
Results pave the way for additional phase 3 investigation
The results presented at ESMO demonstrate not only the safety of the treatment but also nearly halving of the risk of relapse in patients who received the combination of immunotherapies plus chemotherapy (who have the PDL1 marker and non-squamous histology). There is also a benefit in terms of both objective response rates and progression free survival with the combination of nivolumab and relatlimab (1 :1 dose ratio) and chemotherapy. This phase 2 study therefore reveals a clinical benefit and provide proof of concept to initiate further Phase 3 investigation.
Nivolumab (NIVO) plus relatlimab with platinum-doublet chemotherapy (PDCT) vs NIVO + PDCT as first-line (1L) treatment (tx) for stage IV or recurrent NSCLC: results from the randomized phase 2 RELATIVITY-104 study. Pr Nicolas Girard. Proffered paper session NSCLC metastatic. Sous embargo jusqu’au 14 septembre, 9h, heure française.
|
OTHER MADE IN CURIE RESULTS PRESENTED AT ESMO 2024
> Poster session Breast cancer, early stage, 16 septembre : NeoBREASTIM – A Phase 2 Study of Atezolizumab plus RP1 oncolytic immunotherapy in the NeoAdjuvant setting of Triple-Negative Breast Cancer / Association between clinicopathological characteristics and pathological complete response in patients with triple negative breast cancer treated by neoadjuvant chemo-immunotherapy
Ewing sarcoma is a rare form of bone cancer that mainly affects children and young adults, with 20% of metastatic forms at diagnosis. The COMBINAIR3 study, presented at ESMO and coordinated by Dr. Valérie Laurence, medical oncologist, head of the Adolescents and Young Adults Unit at Institut Curie, evaluated a treatment strategy for patients with very high-risk Ewing sarcoma with extrapulmonary metastases, combining induction chemotherapy followed by high-dose consolidation chemotherapy with peripheral stem cell reinjection, local treatment and then maintenance chemotherapy over two years. The results support improved recurrence-free survival rates with few serious side effects. This approach seems to be well suited to treat this rare cancer with metastases. > Poster session Sarcoma, 14 septembre : Multimodal therapy in first line treatment of very high risk Ewing Sarcoma patients: results of the French prospective multicenter COMBINAIR3 phase II trial
The results of the first analysis using the WAYFIND-R platform, an international registry of databases, will be presented by Prof. Christophe Le Tourneau, Head of the Early Clinical Trials Department at Institut Curie. This study, conducted on more than 2,400 patients from 24 different countries, describes the actual use of next-generation sequencing (NGS) and its impact on treatment decisions around the world. This work shows an increase in the use of targeted therapy and immunotherapy after NGS testing, in several types of cancer.
After treatment for localized hormone-sensitive breast cancer, women receive hormone therapy for several years to limit the risk of relapse, a risk that is increased in younger women. Why is this so? One of the possible reasons is the lack of adherence to hormone therapy in this population, but no study has quantified the improvement in prognosis that could be achieved by better adherence. Using data from the French National Health Data System, Dr Elise Dumas, a post-doctoral fellow at the Ecole Polytechnique Fédérale de Lausanne (EPFL), and the Institut Curie analyzed a cohort of 121,852 patients with hormone-dependent early breast cancer (29.9% aged under 50, and 1.8% aged under 34 at diagnosis). The results of this study, presented at the ESMO annual congress, show that strict monitoring of hormone therapy could lead to a recurrence-free survival benefit of 5.6 percentage points at 5 years, in women diagnosed before the age of 34. This work underlines the need for a specific, tailored strategy for this population of young women, and resonates strongly with the research being carried out within the new Women’s Cancers Institute, an IHU co-founded by Institut Curie, Université PSL and Inserm. > Mini oral session: Breast cancer, early stage - Explaining the relationships between age, endocrine therapy persistence and risk of recurrence in hormone-positive early breast cancer : A nationwide cohort study – Saturday 14 September |
In addition to the original results presented at the congress, Institut Curie physicians and researchers are invited to various expert sessions:
- Sebastian Amigorena, CNRS Research Director, Team Leader at Institut Curie and Director of the CellAction (Cell Therapy Acceleration and Innovation) platform at Institut Curie , will organize and lead a symposium: "Beyond coding neoantigens: What else is there for T cells to see"
- The Dr Etienne Brain, medical oncologist at Institut Curie, will be speaking in several special sessions on theGeriatric oncology and in particular the care of elderly patients with breast cancer.
- Sylvie Bonvalot, surgical oncologist, specialist in soft tissue sarcomas at Institut Curie, will speak at a multidisciplinary symposium dedicated to localized sarcomas.
- François-Clément Bidard, medical oncologist and coordinator of breast cancer research at the Institut Curie hospital, will speak at the "congress highlights" session on breast cancer.
- Christophe Le Tourneau, Head of the Early Clinical Trials Department at Institut Curie, is invited to discuss the results presented during an oral session on Developmental therapeutics.
- Dr . Emanuela Romano, medical oncologist, medical director of the Cancer Immunotherapy Center at Institut Curie, will lead two sessions dedicated to the ESMO Clinical Practice Guidelines (1 and 2).
Find the full ESMO 2024 programme online